Dorsal/Ventral Axis
Ventralization
Neurulation: Formation of the Neural Tube
Shortly after convergent extension, the axial mesoderm undergoes neurulation which is the formation of the neural tube from the neural plate. There are three factors that are thought to be important in neural tube formation:
1) Convergent extension of the axial mesoderm that causes the overlying neural plate to fold
2) The change in shape of the neural ectodermal cells
3) Cell adhesion molecules that are expressed such as the Cadherins
This forms the beginnings of the Dorsal/ Ventral axis from what was medial and lateral. This neural tube then pinches off from the surface ectoderm.

| The progression of the Neural Tube from the Neural Plate. Image courtesy of Cassy Ashman |
Sonic Hedgehog Signalling
Once the notochord has formed, it starts to express Sonic Hedgehog (Shh) a secreted signalling molecule that is produced and acts in a concentration dependent manner as a morphogen to cause patterns of gene transcription. In high concentrations, Shh causes neural cells to differentiate into floor plate cells which occupy the ventral midline. These floor plate cells also induce the expression of Shh which diffuses dorsally in a concentration gradient governing D/V fate.
Shh binds to Ptc (Patched) a 7 trans-membrane receptor which normally in the absence of Shh acts to repress Smo another receptor so intracellular Gli genes act as transcriptional repressors. However when Shh bind to Ptc it no longer represses Smo so Gli genes can act as transcriptional activators instead. Shh induces the formation of motor neurons by repressing transcription factors such as msx-1 and Pax-3 and by turning on transcription factors such as ChAT which then cause the differentiation of ventral neurons into motor neurons.
Embryonic Stem Cells
Embryonic stem cells (ES cells) are taken from the inner cell mass of the blastocyst embryo at embryonic day 4-5. They can self renew and are able to differentiate into any of the three primary germ layers therefore are called pluripotent cells.
Because we know exactly what factors are required to form specific tissues, we can experimentally direct ES cells to defined neural fates. Motor neurons can be “made” by first exposing ES cells to “neural” signals and then “posterior” signals and Shh. This process has many connotations with regenerative therapy as these motor neurons are able to integrate once they are placed back into the embryo and can even form functional synapses.
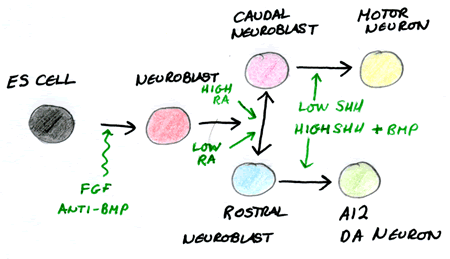 Looking at the differentiation of the axis enables us to understand many defects and diseases in humans. Mice lacking Shh for instance show no ventralization of the neural tube and have holoprosencephaly and cyclopia. These defects have been found in humans and thus we now know they are most likely to arise from malfunctioned Shh signalling.
Looking at the differentiation of the axis enables us to understand many defects and diseases in humans. Mice lacking Shh for instance show no ventralization of the neural tube and have holoprosencephaly and cyclopia. These defects have been found in humans and thus we now know they are most likely to arise from malfunctioned Shh signalling.
| Embryonic Stem Cell Potential Neural Fates. Image Courtesy of Cassy Ashman |
Dorsalization
BMP Signalling
In a similar fashion to Shh in ventral patterning, BMPs form a concentration gradient from dorsal to ventral. BMPs turn on the differential expression of transcription factors and create distinct progenitor domains (dl1-dl6) that differentiate into the dorsal interneurons and commissural neurons.
The Shh and BMP gradients effectively oppose or antagonise each other along the entire dorsal/ ventral axis of the neural tube.
Proliferating Progenitor Cells
If the cells are still proliferating and so are yet to be fully differentiated, they lie in a region closer to the lumen of the neural tube called the sub-ventricular zone. As they differentiate further they migrate towards the outer edge of the neural tube and extend their axons to project to final targets.
Roof Plate Cells and the Neural Crest Cells
As well as a floor plate a roof plate is present in the neural tube that will give rise to neural crest cells. The neural crest cells undergo an epithelial to mesenchymal transition to migrate out of the neural tube. They can be known as the “4th germ layer” as they can further differentiate into different fates including the peripheral nervous system.
No other tissue undergoes such extensive migration as the cells of the neural crest. Both paracrine and receptive cues similar to those that guide axons direct the neural crest cells. These include Fibronectin and Ephrin ligands.

|
The “4th germ layer” of the Neural Crest. Image courtesy of Cassy Ashman |
