Anterior/ Posterior Axis
The neural tube regionalises into a distinct A/P axis when it is still a long tube and before the flexures develop. Most of the neural tube produces the spinal cord however the rostral (most anterior) end swells to produce three primary brain vesicles; the forebrain (prosencephalon), the midbrain (mesencephalon) and the hindbrain (rhombencephalon). These primary brain vesicles then give rise to new structures. The forebrain produces the telecephalon and the diencephalon. The diencephalon induces the optic vesicles which produce the neural retina and lens. The hindbrain produces the metencephalon which forms the cerebellum and pons, and it also produces the myelencephalon which produces the medulla oblongata. The hindbrain or rhombencephalon also becomes divided into regularly spaced sections called rhombomeres divided by distinct boundaries. The midbrain however remains as it is undivided into further sub-compartments.
The tissue uses a distinct model to differentiate between what is “hindbrain” and what is “forebrain” and this is known as the activation-transformation model. It works by first activating the neural tissue with anterior qualities and then goes on to transform some of it into posterior hindbrain and spinal cord.
Before gastrulation the cells of the organiser secrete BMP Inhibitors that induce the neural tissue that has an anterior forebrain character. Only the portions of the axial mesoderm that involutes first maintain BMP inhibitor expression (chordin and noggin). Meanwhile posteriorly, the signals that are proposed to posteriorise the embryo (FGFs, retinoic acid and Wnts) act as morphogens and form gradients from the posterior embryo to anteriorly.
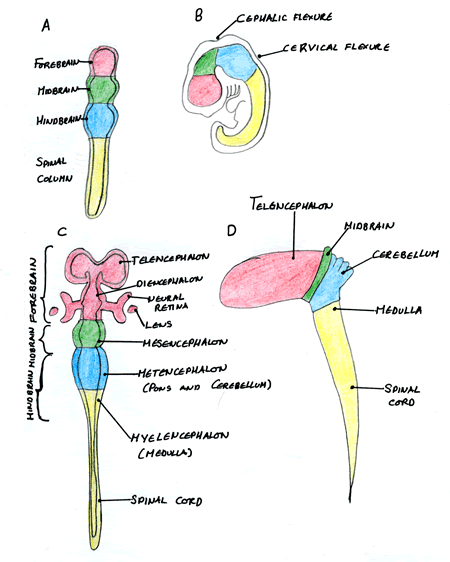
| A) The 3 primary brain vesicles and B) their positions in the early embryo. C) and D) show the development of the primary brain vesicles into further structures. Image courtesy of Cassy Ashman |
Posteriorising Factors: Retinoic Acid and Wnts
Retinoic acid works by transversing the membrane and entering the cell cytoplasm and nucleus to alter gene transcription but works in a concentration dependent manner to do so. The lower levels of retinoic acid anteriorly permit the anterior hox genes being expressed whilst the higher levels of retinoic acid posteriorly repress these anterior hox genes but activate the posterior hox genes.
Wnts unlike retinoic acid are associated with the cell membrane and diffuse limited distances from the cells that produce them. They bind to a seven trans-membrane domain receptor called frizzled and once bound it causes an intracellular β-catenin to associate with Axin, APC and SSH-3B in a complex that normally is only transient in its formation. This results in the accumulation of β-catenin which acts in the nucleus to activate target genes.
Hox Genes
The Hox genes are “homeobox” containing genes that were first identified in the Drosophila embryo as the homeotic selector genes that decide certain body areas’ fate along the A/P axis. Work in the late 80’s found vertebrate homologues of these genes expressed in a nested fashion along the A/P axis.
The Hox genes are activated by a particular threshold concentration of the posteriorising signals and as a result different regions of the neuraxis come to express different Hox genes. This Hox code is vital for the differentiation of all the discrete spinal nerves along the A/P axis for example Hox a1 in rhombomere R5 produces the abducens nerve in this region.
Midbrain Formation
The midbrain is formed at the inter-phase between the forebrain and the hindbrain and gives rise to the formation of the tectum and cerebellum. Experimental evidence has shown if you fuse hindbrain tissue into the forebrain a new boundary is formed and there is an induction of an ectopic midbrain.
The signalling factors that are important in midbrain formation are Otx2 which is produced and maintained in the forebrain whilst Gbx2 is expressed more posteriorly. Where they meet a new organiser region is formed called the isthmus and it is this region that is responsible for the expression of transcription factors that cause further differentiation of this region.
Forebrain Formation
In the prosomeric model of forebrain development it is proposed that there are longitudinal and transverse patterns of gene expression that subdivided the neural tube into a grid of different identities. The prosencephalon or forebrain can be divided into six prosomeres from caudal to rostral (P1 to P6).
An important set of transcription factors involved in differentiation of the forebrain are called the Pax genes which have a homeodomain region and a paired box. There are 9 in total and they all expressed in the nervous system except for Pax 1 and Pax 9. For example, Pax 6 is responsible for human eye development.
